

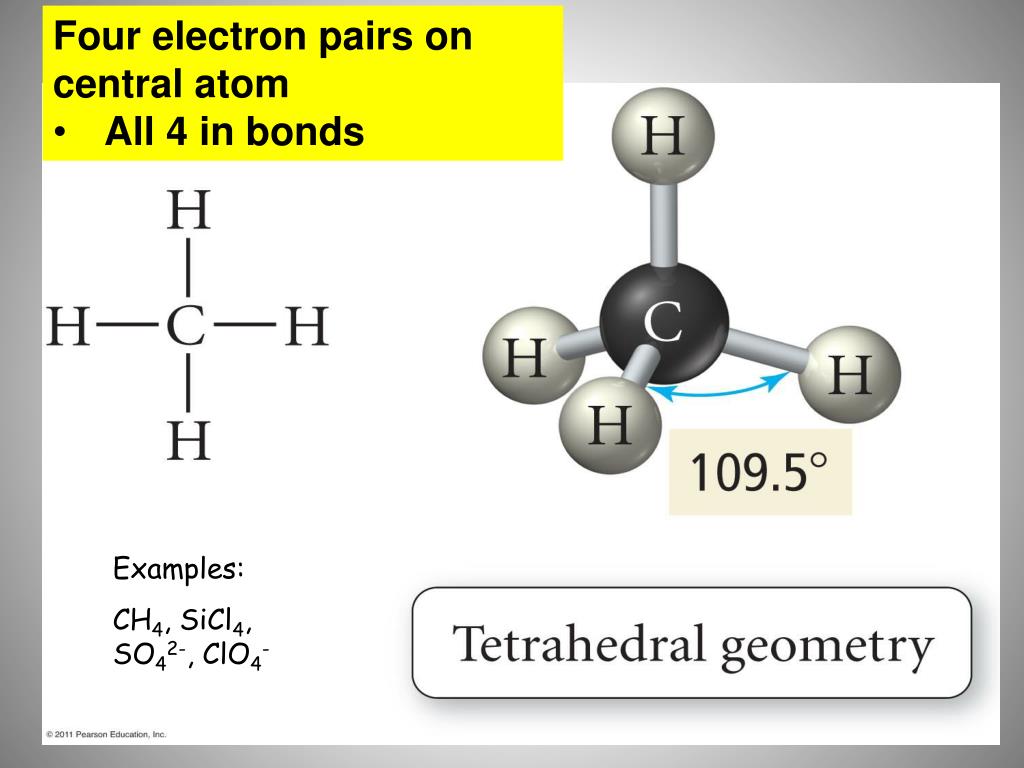
The correct Lewis structure with dash and wedge notation for CH 4 is depicted on the right in Figure 1. Bonds drawn with a solid triangle, or wedge, represent bonded atoms coming out of the page/screen towards the observer bonds drawn with dashed triangle, or dash, represent bonded atoms going into the page/screen away from the observer. Bonds drawn as simple lines are located in the plane of the page or screen. Chemists use dash and wedge notation to draw 3D molecules. Representing three dimensions on 2D paper (or computer screen) is challenging. The Lewis structure is misleading because it depicts methane as a planar ‘plus-shaped’ molecule, yet we know that methane is a non-planar, tetrahedral molecule. Methane (CH 4) depicted by a Lewis structure (left) and dash and wedge notation (right). Depicted on the left in Figure 1 is the Lewis structure for methane (CH 4) showing the central carbon atom singly bonded to four hydrogen atoms: Figure 1. The Lewis structure gives us meaningful information about the bonds between atoms, but Lewis structures do not depict how the molecule exists in three-dimensions. | Key Concepts and Summary | Glossary | End of Section Exercises | Lewis Structures with Wedge-Dash Notation | Lewis Structures with Wedge-Dash Notations |
We can use the following notations when examining a Lewis structure of a molecule.Į = non-bonding electron pairs of the central atom This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional space and this gives the molecules their shape. VESPR stands for valence shell electron pair repulsion. It applies a theory called VESPR for short. Molecular geometry is a way of describing the shapes of molecules. Similar logic applies to all the shapes, you just have to remember which "spoke" will be taken up by an electron pair. Once there are any electron pairs, one spoke of the original shape gets "eaten up": for example, a #AX_4E_2# is an octahedron shape, but the two "spokes" are taken up by electron pairs, so you're left with just the square-a square planar shape. #6#: octahedron (a flat square with two "spokes") #5#: trigonal bipyramid (a trigonal planar shape with two "spokes") #3#: trigonal plane (a flat equilateral-triangle-looking shape) As it has a VSEPR shape #AX_5E_0# it is a trigonal bipyramid.Įach steric number has a same "basic shape":
ELECTRON PAIR GEOMETRY PLUS
Its steric number is #5# due to the #5# bonded atoms to the central #S# atom plus #0# lone electron pairs. Thus, it is in the form #AX_3E_1#, which forms a trigonal pyramidal shape. #N#, the central atom, has a steric number of #4#, calculated by the #3# atoms it's bonding with #+1# lone pair. This is the total number of electron pairs and bonds with other atoms. Find the central molecules' steric numbers.


 0 kommentar(er)
0 kommentar(er)
